Rapid Diagnostic Reagents
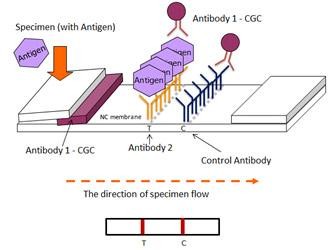
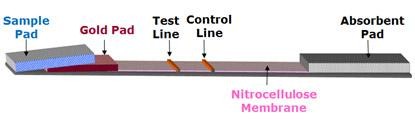
Rapid diagnostic reagents utilize immunoassay technology and chromatographic principles to detect the presence of specific antibodies or antigens in a specimen. This is achieved by immobilizing target antibodies or antigens onto a nitrocellulose membrane and using nano-scale colloidal gold particles as the visual marker for results.
A. Colloidal Gold Preparation
Colloidal gold particles are widely used as visual markers in diagnostic reagents due to the following advantages:
1. Simple synthesis methods.
2. Easy dispersion into colloidal form and relative stability after synthesis.
3. High stability when conjugated with antibodies or antigen molecules.
Excelsior Bio-System (EBS) produces colloidal gold particles with uniform size and stable quality using chemical synthesis methods.
B. Reagent Development
EBS applies immunological principles to test potential antibodies or antigens to identify optimal combinations. The company develops different lateral flow immunochromatographic test reagents tailored to diverse diagnostic needs.
C. Performance Validation and Quality Control
EBS establishes strict performance evaluation standards throughout the development process to ensure product efficacy and quality. After the prototype is developed, extensive performance validation tests are conducted to confirm the functionality, stability, and convenience of the reagents. The tests include:
-
Interference Testing:
- Evaluate the impact of potential interfering substances present in real-world specimens to ensure they do not affect reagent performance or stability.
-
Accelerated and Stability Testing:
- Monitors the relationship between temperature and time during development to estimate the shelf life and ensure the stability of diagnostic reagents within the effective period.
D. Antibody and Antigen Production Technology
To enable the flexible development of various diagnostic reagent products and achieve raw material independence, EBS has developed comprehensive antibody and antigen production technologies. These are tailored to the specific needs and characteristics of target products and follow these procedures:
-
Antigen Recombinant Protein Design:
- Utilizes molecular biology principles to design recombinant proteins for target antigens.
-
Gene Sequence Identification:
- Searches online gene databases to locate specific antigen gene sequences.
-
Gene Transfection and Antigen Expression:
- Inserts target genes into new vectors through gene transfection technology and transfers them into specific expression cells for large-scale production of recombinant antigen proteins.
EBS has established a robust recombinant protein purification platform. The process includes precipitation, gel chromatography, ion exchange chromatography, and affinity chromatography to enhance protein purity and usability. These purified antigens are applied in developing monoclonal and polyclonal antibodies, which are subsequently utilized in creating diagnostic products.